Mid-Atlantic Coastal Ecosystems
How Regional Conditions Influence Saturation State
Many marine organisms, including shellfish, mollusks and plankton, make calcium carbonate skeletal structures in the form of aragonite or calcite. The saturation state of calcium carbonate is a measure of whether the skeletal structures will dissolve (low saturation state) or form (high saturation state).
Offshore vs. Nearshore Conditions
Acidification works to decrease saturation state. The nearshore waters of the Mid-Atlantic exhibit relatively low aragonite saturation states (blue regions on the map), while further offshore the warmer, more saline and more strongly buffered waters have relatively higher saturation states (orange and red on the map). As freshwater flows from Mid-Atlantic estuaries into nearshore regions, excess nutrients from fertilizer, wastewater treatment, and stormwater runoff lead to coastal eutrophication and exacerbate acidification.
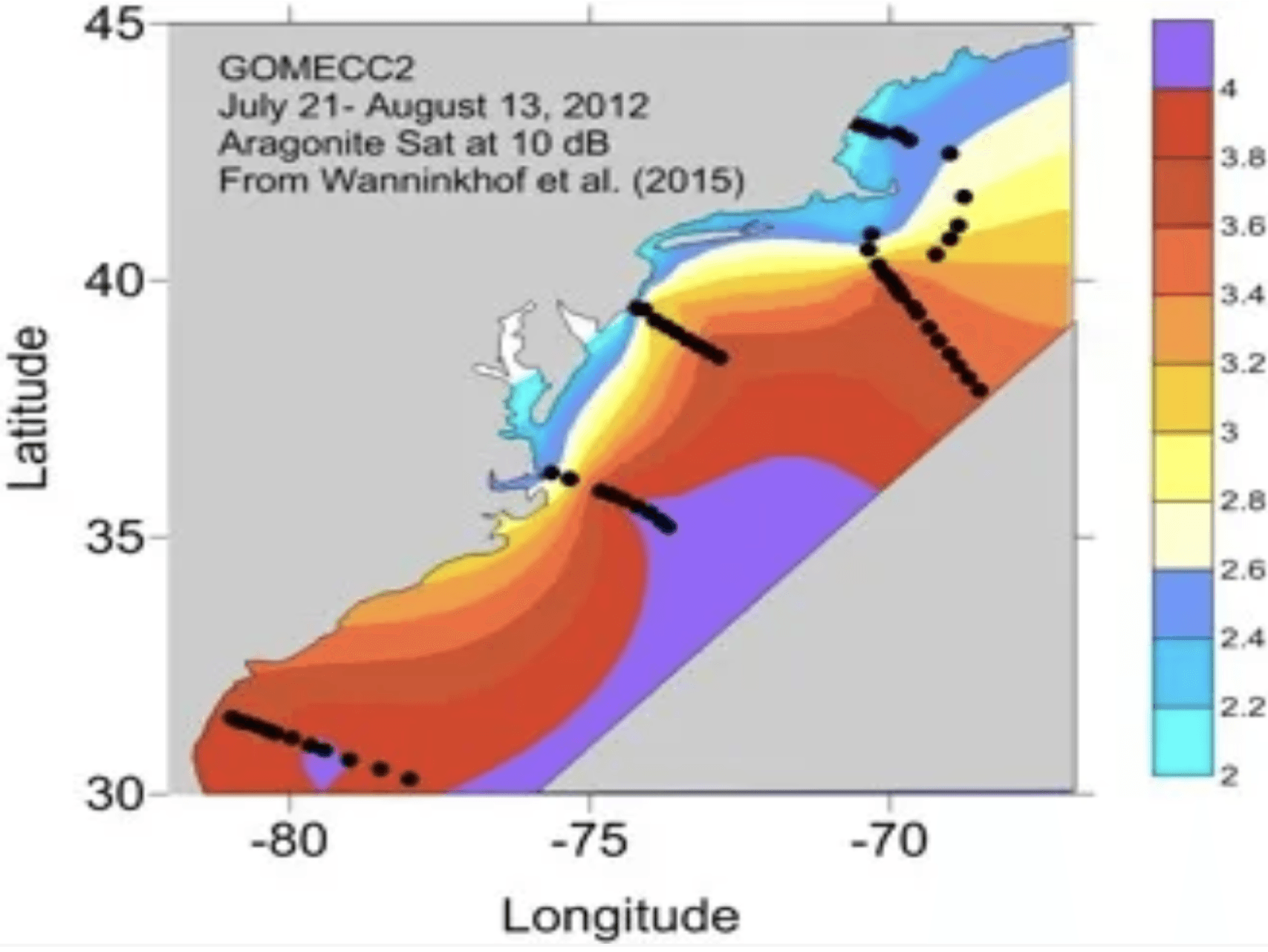
The above map depicts the aragonite saturation in the Mid-Atlantic. Low saturation state will result in the dissolution the shells or skeletal structures of many marine organisms, while high saturation states will allow shells to form.
In winter, saturation state is suppressed throughout the region due to cooling of the water, and the respiration or remineralization of the algal material that is produced during the spring and summer seasons. The combination of these processes elevate CO2 and decrease pH. This natural seasonality complicates assessments of changes due to anthropogenic acidification and highlights the need for sustained monitoring of regional ocean chemistry.


References
Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.