
Total Alkalinity (TA)
TA Sites
About
Alkalinity is the measurement of the ability of a solution (i.e. seawater) to accept positively charged ions (H+) and bind them to a negatively charged base ion or molecule, thus neutralizing the acid. Alkalinity is important as it is a measurement of the ocean’s ability to resist acidification.
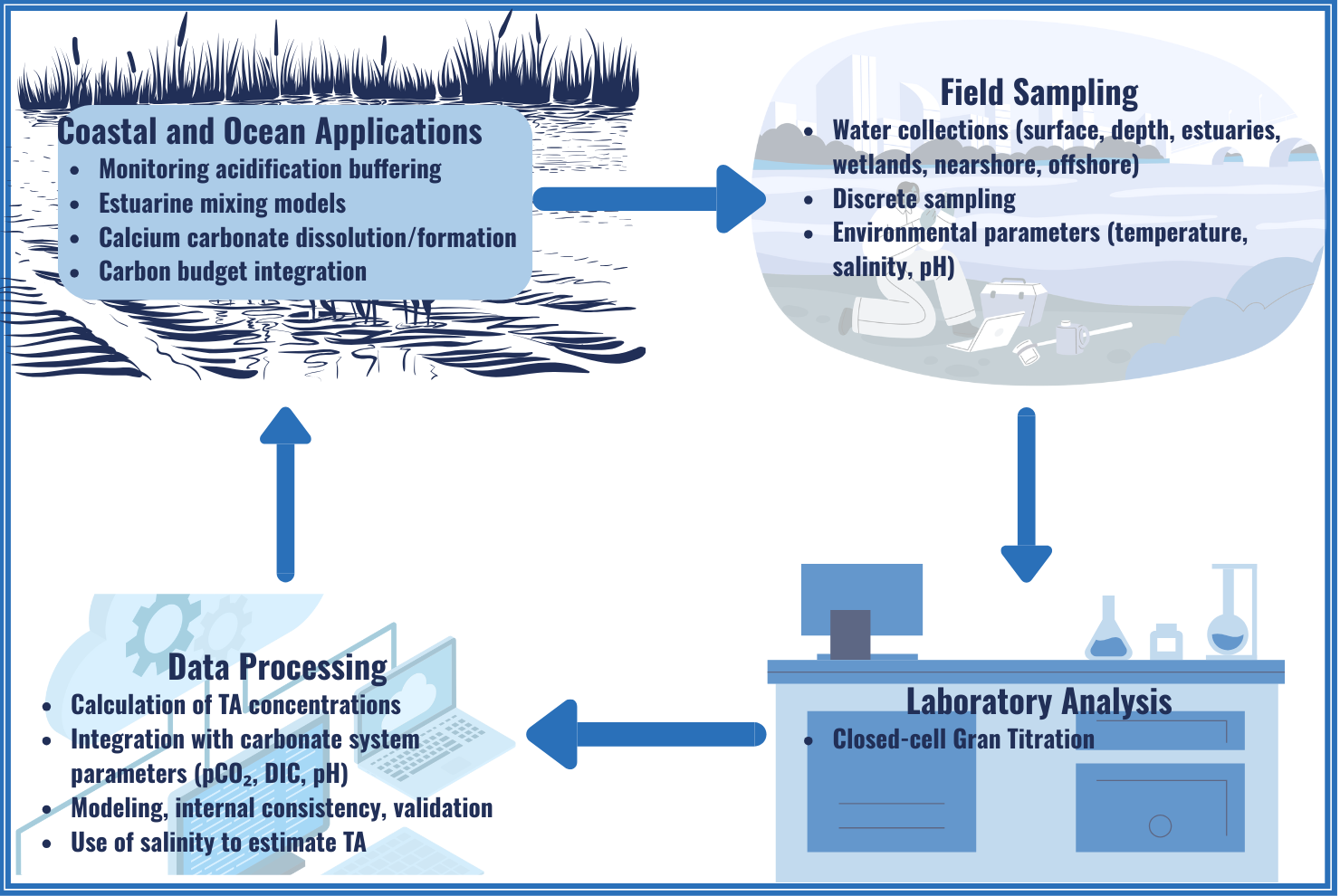
Photo: Janet J. Reimer
Total Alkalinity Buffers Ocean Acidification
Total Alkalinity (TA) refers to the difference between the negatively (Cl-, SO42-, Br-, and F) and positively (Na+, Mg2+,Ca2+, K+ and Sr2+) charged ions in water. Together these ions have a net positive charge because overall there are more positively charged ions in water. The overall charge of the water is neutralized by the HCO₃⁻ and CO₃²⁻ ions. HCO₃⁻ and CO₃²⁻ are defined as carbonate alkalinity (CA). The ions that influence TA are conservative, meaning that their concentrations are consistent in water. CA is the main factor in determining alkalinity. If the CA is high enough, the HCO₃⁻ and CO₃²⁻ ions can act to buffer the water from dramatic changes in pH accepting hydrogen ions.
High CO₂ Lowers pH and Reduces Buffering
The increased forcing of CO₂ (high pCO₂ ) into the water is resulting in lower pH and could alter carbon speciation (see Dissolved Inorganic Carbon section above) which can reduce the water’s ability to buffer changes in pH. In addition, estuaries and other coastal waters typically have lower alkalinity due to dilution from freshwater sources, compared to the open ocean.
Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.
