
Partial Pressure of Carbon Dioxide (pCO₂)
pCO₂ Sites
About
Partial Pressure of Carbon Dioxide (pCO₂) refers to the idea that in a mixture of gases, the total pressure exerted by that mixture is equal to the sum of the individual pressures, or partial pressures, of the gases. According to Henry’s law, the concentration of a gas dissolved in a liquid is directly proportional to the partial pressure of the gas above the liquid. As atmospheric CO₂ levels have increased from human activities, the ocean has responded by absorbing CO₂ in order to maintain equilibrium between the air and seawater.
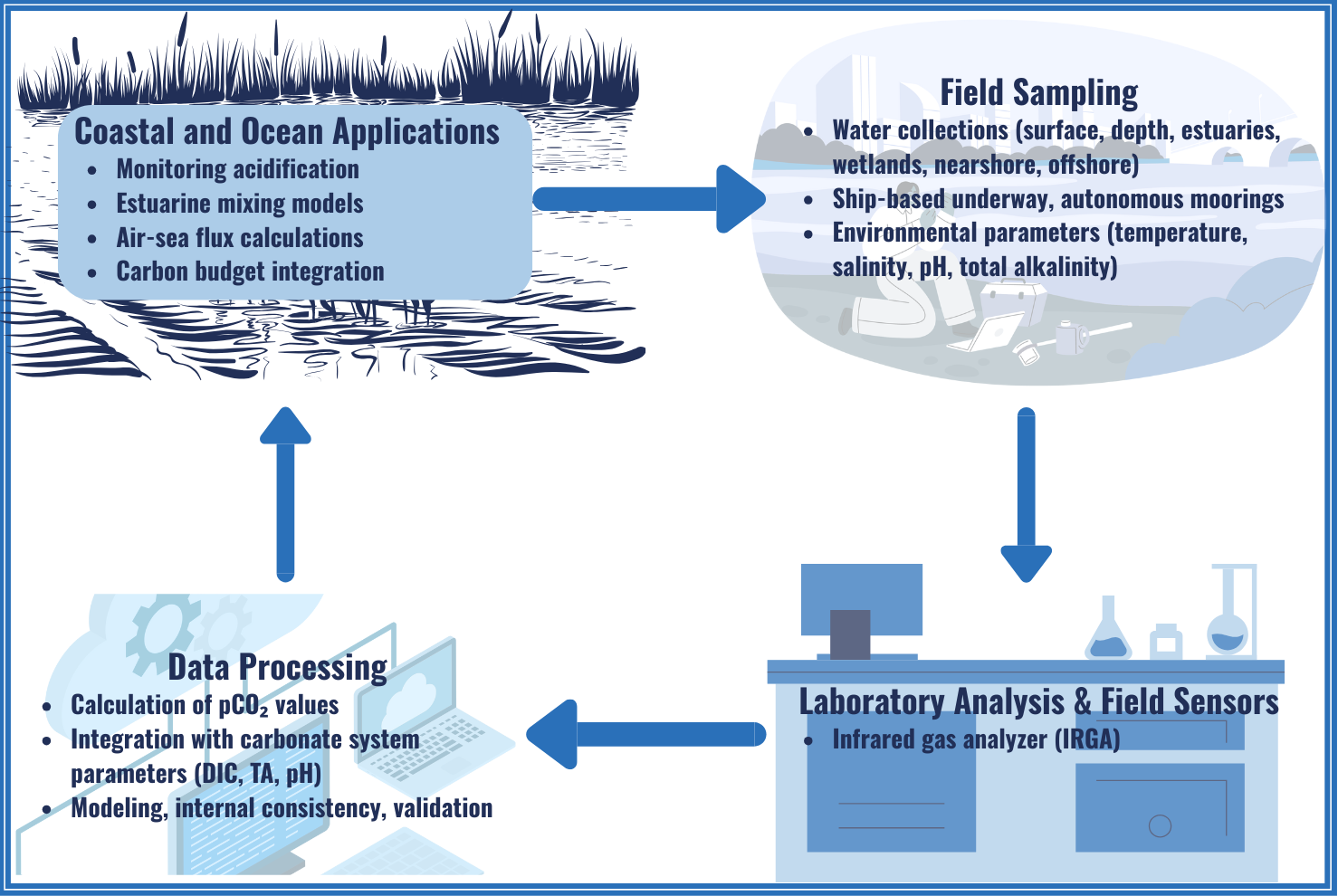
Photo: Janet J. Reimer
Estuaries Release CO₂ Like Soda
Often, estuaries and coastal areas have very high concentrations of dissolved CO₂ compared to the overlying atmosphere. High estuarine pCO₂ is typically the result of biological activity, in which CO₂ production (through respiration, by heterotrophs) exceeds CO₂ consumption (through photosynthesis, by autotrophs). Located at the land-sea interface, estuaries receive organic matter and nutrients from the land, providing extra fuel for heterotrophs. When an estuary’s pCO₂ is higher than the pCO₂ of the air above it, CO₂ leaves the water and enters the atmosphere.
Think about a can of soda. Before it is opened there is a headspace at the top of the can that is pressurized with CO₂. That high CO₂ partial pressure of the headspace ensures that the CO₂ in the soda remains dissolved. When the can is opened, the CO₂ in the headspace encounters the outside atmosphere (which has a relatively low concentration of CO₂). The result is that the soda fizzes and bubbles float to the surface, releasing previously dissolved CO₂ into the atmosphere.


Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.

