
Dissolved Inorganic Carbon (DIC)
DIC Sites
About
When carbon dioxide enters the ocean, it reacts with water to create four specific chemical forms called species: aqueous carbon dioxide (CO₂(aq)), carbonic acid (H₂CO₃), bicarbonate (HCO₃⁻), and carbonate (CO₃²⁻). Collectively these species are known as dissolved inorganic carbon (DIC).
A series of reactions take place simultaneously until an equilibrium is reached among the four species. When CO₂ is dissolved in water, either from the atmosphere or from respiration from coastal organisms, it reacts with H₂O to produce H₂CO₃.
CO2(aq) + H2O <-> H₂CO₃
H₂CO₃ <-> HCO₃⁻ + H⁺: H₂CO₃ breaks down to release a hydrogen ion
HCO₃⁻ <-> CO₃²⁻ + H⁺: If pCO2 increases, HCO₃⁻ loses another H⁺

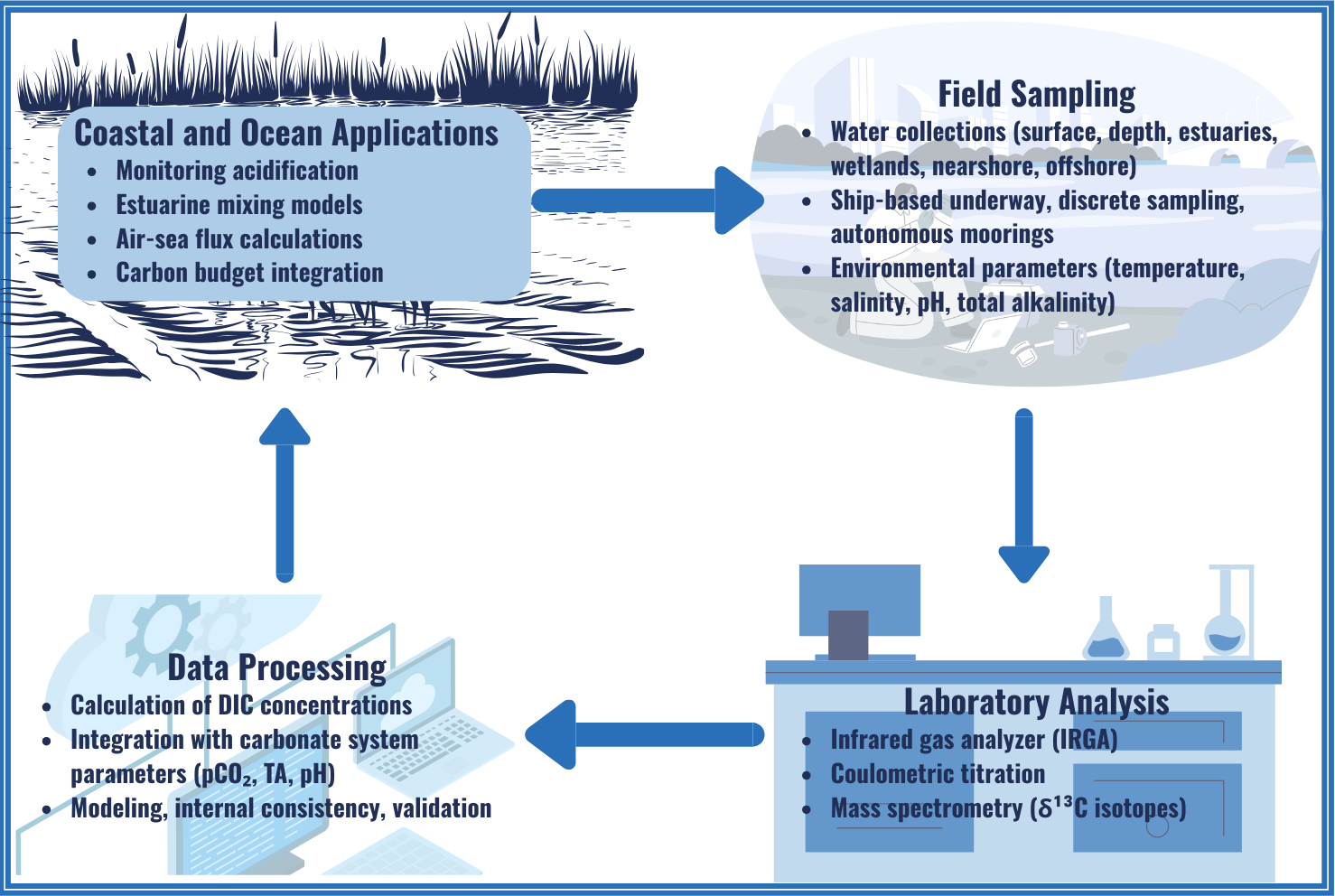
Photo: Janet J. Reimer
More CO₂ Means More Acidification
These reactions occur simultaneously until an equilibrium, or balance, is reached. If more CO2 is added (a rise in pCO2) H₂CO₃ breaks down into HCO₃⁻ and CO₃²⁻ and more H⁺ ions get released, lowering the pH and increasing acidification.


Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.

